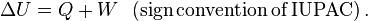Internal energy is all the energy of a system that is associated with its microscopic components—atoms and molecules—when viewed from a reference frame at rest with respect to the center of mass of the system
It is associated to the degree of Freedom of an atom or group of atoms.These degree of freedom can be translational,rotational ,vibrational which we will deal later in this page.
Thermal energy can be interpreted as that part of the internal energy associated
with random motion of molecules and, therefore, related to temperature. Bond energy is the
intermolecular potential energy. Therefore,{ Internal energy} ={ thermal energy+ bond energy} Although this breakdown is presented here for clarification with regard to other books, we will not use these terms because there is no need for them.
Heat is defined as the transfer of energy across the boundary of a system due to a temperature difference between the system and its surroundings.
When you heat a substance, you are transferring energy into it by placing it in contact with surroundings that have a higher temperature. Such is the case, for example, when you place a pan of cold water on a stove burner. The burner is at a higher temperature than the water, and so the water gains energy. We shall also use the term heat to represent the amount of energy transferred by this method.
Work and Heat in Thermodynamic Processes
In thermodynamics, we describe the state of a system using such variables as pressure, volume, temperature, and internal energy. As a result, these quantities belong to a category called state variables. For any given configuration of the system, we can identify values of the state variables. (For mechanical systems, the state variables include kinetic energy K and potential energy U.) A state of a system can be specified only if the system is in thermal equilibrium internally. In the case of a gas in a container, internal thermal equilibrium requires that every part of the gas be at the same pressure and temperature.
A second category of variables in situations involving energy is transfer variables. These variables are zero unless a process occurs in which energy is transferred across the boundary of the system. Because a transfer of energy across the boundary represents a change in the system, transfer variables are not associated with a given state of the system, but with a change in the state of the system. In the previous sections, we discussed heat as a transfer variable. For a given set of conditions of a system, there is no defined value for the heat. We can only assign a value of the heat if energy crosses the boundary by heat, resulting in a change in the system. State variables are characteristic of a system in thermal equilibrium. Transfer variables are characteristic of a process in which energy is transferred between a system and its environment.
In this section, we study another important transfer variable for thermodynamic systems—work.
Consider a gas contained in a cylinder fitted with a movable piston as shown above.At equilibrium, the gas occupies a volume V and exerts a uniform pressure P on the cylinder’s walls and on the
piston. If the piston has a cross-sectional area A, the force exerted by the gas on the piston
is F = PA.
Now let us assume that we push the piston inward and compress the gas
quasi-statically, that is, slowly enough to allow the system to remain essentially in
thermal equilibrium at all times. As the piston is pushed downward by an external
force F =-F ˆj through a displacement of dr = dyˆj in vector notation since the displacement is to the positive y axis direction.Look at the above figure b.
The work done on the gas is
where we have set the magnitude F of the external force equal to PA because the piston
is always in equilibrium between the external force and the force from the gas.
For this discussion, we assume the mass of the piston is negligible. Because Ady is the change in volume of the gas dV, we can express the work done on the gas as
If the gas is compressed, dV is negative and the work done on the gas is positive. If the gas expands, dV is positive and the work done on the gas is negative. If the volume remains constant, the work done on the gas is zero. The total work done on the gas as its volume changes from Vi to Vf is given by the integral of Equation As show below To evaluate this integral, one must know how the pressure varies with volume duringthe process.
In general, the pressure is not constant during a process followed by a gas, but
depends on the volume and temperature. If the pressure and volume are known at
each step of the process, the state of the gas at each step can be plotted on a graph
called a PV diagram, as in Figure below
This type of diagram allows us to visualize a process through which a gas is progressing. The curve on a PV diagram is called the path taken between the initial and final states.
Note that the integral in Equation
is equal to the area under a curve on a PV diagram. Thus, we can identify an important use for PV diagrams
As Figure in blue suggests, for our process of compressing a gas in the cylinder, the
work done depends on the particular path taken between the initial and final states:
To illustrate this important point, consider several different paths connecting i and f as shown below
In the process depicted in Figure(a)the volume of the gas is first reduced from Vi to Vf at constant pressure Pi and the pressure of the gas then increases from Pi to Pf by heating at constant volume Vf . The work done on the gas along this path is -Pi(Vf - Vi).In Figure b, the pressure of the gas is increased from Pi to Pf at constant volume Vi and then the volume of the gas is reduced from Vi to Vf at constant pressure Pf . The work done on the gas is -Pf(Vf - Vi), which is greater than that for the process described in Figure a.It is greater because the piston is moved through the same displacement by a larger force than for the situation
in Figure a.
Finally, for the process described in Figure c, where both P and V change continuously, the work done on the gas has some value intermediate between the values obtained in the first two processes. To evaluate the work in this case, the function P(V ) must be known, so that we can evaluate the integral in the equation below:
The energy transfer Q into or out of a system by heat also depends on the process. Consider the situations depicted in Figure below
In each case, the gas has the same initial volume, temperature, and pressure, and is assumed to be ideal. In Figure a, the gas is thermally insulated from its surroundings except at the bottom of the gas-filled region, where it is in thermal contact with an energy reservoir. An energy reservoir is a source of energy that is considered to be so great that a finite transfer of energy to or from the reservoir does not change its temperature. The piston is held at its initial position by an external agent—a hand, for instance. When the force holding the piston is reduced slightly, the piston rises very slowly to its final position. Because the piston is moving upward, the gas is doing work on the piston. During this expansion to the final volume Vf , just enough energy is transferred by heat from the reservoir to the gas to maintain a constant temperature Ti .
Now consider the completely thermally insulated system shown in Figure b above. When the membrane is broken, the gas expands rapidly into the vacuum until it occupies a volume Vf and is at a pressure Pf . In this case, the gas does no work because it does not apply a force—no force is required to expand into a vacuum. Furthermore, no energy is transferred by heat through the insulating wall.The initial and final states of the ideal gas in Figure a above are identical to the initial and final states in Figure b above, but the paths are different. In the first case, the gas does work on the piston, and energy is transferred slowly to the gas by heat. In the second case, no energy is transferred by heat, and the value of the work done is zero. Therefore, we conclude that energy transfer by heat, like work done, depends on the initial, final, and intermediate states of the system. In other words, because heat and work depend on the path, neither quantity is determined solely by the end points of a thermodynamic process.
The First Law of Thermodynamics
The first law of thermodynamics for a closed system was expressed in two ways by Clausius. One way referred to cyclic processes and the inputs and outputs of the system, but did not refer to increments in the internal state of the system. The other way referred to any incremental change in the internal state of the system, and did not expect the process to be cyclic. A cyclic process is one that can be repeated indefinitely often and still eventually leave the system in its original state.
In each repetition of a cyclic process, the work done by the system is proportional to the heat consumed by the system. In a cyclic process in which the system does work on its surroundings, it is necessary that some heat be taken in by the system and some be put out, and the difference is the heat consumed by the system in the process. The constant of proportionality is universal and independent of the system and was measured by James Joule in 1845 and 1847, who described it as the mechanical equivalent of heat.
For a closed system, in any process, the change in the internal energy is considered due to a combination of heat added to the system and work done by the system. Taking
 as a change in internal energy, one writes
as a change in internal energy, one writes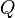 and
and  are quantities of heat supplied to the system by its surroundings and
of work done by the system on its surroundings, respectively. This sign
convention is implicit in Clausius' statement of the law given above,
and is consistent with the use of thermodynamics to study heat engines, which provide useful work that is regarded as positive.
are quantities of heat supplied to the system by its surroundings and
of work done by the system on its surroundings, respectively. This sign
convention is implicit in Clausius' statement of the law given above,
and is consistent with the use of thermodynamics to study heat engines, which provide useful work that is regarded as positive.In modern style of teaching science, however, it is conventional to use the IUPAC convention by which the first law is formulated in terms of the work done on the system. With this alternate sign convention for work, the first law for a closed system may be written:
When a system expands in a fictive quasistatic process, the work done by the system on the environment is the product, P dV, of pressure, P, and volume change, dV, whereas the work done on the system is -P dV. Using either sign convention for work, the change in internal energy of the system is:
Work and heat are expressions of actual physical processes of supply or removal of energy, while the internal energy U is a mathematical abstraction that keeps account of the exchanges of energy that befall the system. Thus the term heat for Q means "that amount of energy added or removed by conduction of heat or by thermal radiation", rather than referring to a form of energy within the system. Likewise, the term work energy for W means "that amount of energy gained or lost as the result of work". Internal energy is a property of the system whereas work done and heat supplied are not. A significant result of this distinction is that a given internal energy change ΔU can be achieved by, in principle, many combinations of heat and work.
When we introduced the law of conservation of energy , we stated that the change in the energy of a system is equal to the sum of all transfers of energy across the boundary of the system. The first law of thermodynamics is a special case of the law of conservation of energy that encompasses changes in internal energy and energy transfer by heat and work. It is a law that can be applied to many processes and provides
a connection between the microscopic and macroscopic worlds.
suppose that a system undergoes a change from an initial state to a final state. During this change, energy transfer by heat Q to the system occurs, and work W is done on the system As an example, suppose that the system is a gas in which the pressure and volume change from Pi and Vi to Pf and Vf . If the quantity Q + W is measured for various paths connecting the initial and final equilibrium states, we find that it is the same for all paths connecting the two states. We conclude that the quantity Q + W is determined completely by the initial and final states of the system, and we call this quantity the change in the internal energy of the system. Although Q and W both depend on the path, the quantity Q+ W is independent of the path. If we use the symbol Eint to represent the internal energy, then the change in internal energy delEint can be expressed as
where all quantities must have the same units of measure for energy. Equation above is known as the first law of thermodynamics. One of the important consequences of the first law of thermodynamics is that there exists a quantity known as internal energy whose value is determined by the state of the system. The internal energy is therefore a state variable like pressure, volume, and temperature.
When a system undergoes an infinitesimal change in state in which a small amount
of energy dQ is transferred by heat and a small amount of work dW is done, the internal
energy changes by a small amount dEint. Thus, for infinitesimal processes we can
express the first law as a mathematical differential way
The first law of thermodynamics is an energy conservation equation specifying that the only type of energy that changes in the system is the internal energy E int. Let us investigate some special cases in which this condition exists. First, consider an isolated system—that is, one that does not interact with its surroundings.
In this case, no energy transfer by heat takes place and the work done on the system is zero; hence, the internal energy remains constant. That is, because Q = W = 0,it follows that delE int =0,
and thus Eint, i = Eint, f .We conclude that the internal energy Eint of an isolated system remains constant.
Next, consider the case of a system (one not isolated from its surroundings) that is taken through a cyclic process—that is, a process that starts and ends at the same state. In this case, the change in the internal energy must again be zero, because Eint is a state variable, and therefore the energy Q added to the system must equal the negative of the work W done on the system during the cycle. That is, in a cyclic process,
On a PV diagram, a cyclic process appears as a closed curve. (The processes described in
Figure here
are represented by open curves because the initial and final states differ.) It can be shown that in a cyclic process, the net work done on the system per cycle equals the area enclosed by the path representing the process on a PV diagram.
Some Applications of the First Lawof Thermodynamics:
The first law of thermodynamics that we discussed in the preceding section relates the changes in internal energy of a system to transfers of energy by work or heat. In this section, we consider applications of the first law to processes through which a gas is taken. As a model, we consider the sample of gas contained in the piston–cylinder apparatus in figure shown below
This figure shows work being done on the gas and energy transferring in by heat, so the internal energy of the gas is rising. In the following discussion of various processes, refer back to this figure and mentally alter the directions of the transfer of energy so as to reflect what is happening in the process.
Before we apply the first law of thermodynamics to specific systems, it is useful to
first define some idealized thermodynamic processes. An adiabatic process is one
during which no energy enters or leaves the system by heat—that is, Q = 0. An adiabatic
process can be achieved either by thermally insulating the walls of the system,
such as the cylinder in as shown above or by performing the process rapidly, so that there
is negligible time for energy to transfer by heat. Applying the first law of thermodynamics
to an adiabatic process, we see that
From this result, we see that if a gas is compressed adiabatically such that W is positive,
then delEint is positive and the temperature of the gas increases. Conversely, the temperature
of a gas decreases when the gas expands adiabatically.Adiabatic processes are very important in engineering practice. Some common examples are the expansion of hot gases in an internal combustion engine, the liquefaction of gases in a cooling system, and the compression stroke in a diesel engine as shown at the heading figure.
I will come with the rest of topic in my next page till my subscribe to my page here.
Advance Mathematical research by Md Tauseef Ibrahim/Abraham Malik
Electronics And Communication by Md Tauseef Ibrahim/Abraham malik
- http://electronicsandcommunicationadvancedma.blogspot.in/2015/03/3g-networksorthogonal-frequency.html
- http://electronicsandcommunicationadvancedma.blogspot.in/2015/03/3g-networksorthogonal-frequency.html